In the vast field of nuclear physics, the Bohr model stands as a fundamental concept that has revolutionized our understanding of the structure of atoms. Of the numerous elements present in the periodic table, antimony, with its enigmatic properties, provides a fascinating subject for exploration within the framework of the Bohr model.
Bohr Model Unveiled:
Proposed by Danish physicist Niels Bohr in 1913, the Bohr model provided an unprecedented explanation of atomic structure. At its core, the model portrays the atom as a miniature solar system, with electrons orbiting the nucleus, just as planets orbit the Sun. However, unlike classical mechanics, where planets orbit in a continuous manner, Bohr introduced quantized energy levels, suggesting that electrons can only occupy specific, distinct orbits around the nucleus.
Antimony: A look at its elemental nature:
Antimony, a shiny gray metalloid, occupies an interesting place in the periodic table. With atomic number 51, it sits just below arsenic and above tellurium. Its properties span the spectrum of metals and nonmetals, rendering it a unique element with diverse applications ranging from flame retardants to semiconductor materials.
Atomic structure of antimony through Bohr's lens:
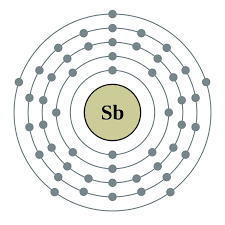
When we delve into the atomic structure of antimony through the lens of the Bohr model, we encounter a fascinating interplay of electrons and energy levels. At its core, the antimony atom consists of a nucleus containing 51 protons and a variable number of neutrons, depending on its isotope. There is a series of electron shells around this nucleus, each of which has its own energy level.
In the Bohr model, electrons in an atom occupy specific energy levels or shells, which are represented by quantum numbers. These shells are labeled with principal quantum numbers, which are represented by the letter "n" and range from 1 to infinity. The first shell closest to the nucleus has the lowest energy level and can hold a maximum of 2 electrons. Later shells can accommodate more electrons with higher energy levels.
Electron configuration of antimony:
For antimony, the electron configuration follows the pattern determined by the Bohr model. With 51 electrons, antimony's electron configuration can be broken down as follows:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p³
This configuration reflects the arrangement of electrons within different energy levels or electron shells of the antimony atom. Each superscript represents the number of electrons occupying a particular subshell.
Role of valence electrons:
Valence electrons, which occupy the outermost shell of an atom, play an important role in determining the chemical properties of an element. In the case of antimony, the outermost shell is the fifth shell (n=5), which contains 5 electrons (5s² 5p³). These valence electrons participate in chemical bonding and interactions, which affect the behavior of antimony in compounds and reactions.
Applications and Implications:
Understanding the atomic structure of antimony through the Bohr model not only sheds light on its fundamental properties but also paves the way for various applications. From its use in alloys to its role in electronics and medicine, antimony's unique characteristics find diverse practical implementations.
Furthermore, the discovery of antimony within the framework of the Bohr model contributes to our broader understanding of nuclear physics. This serves as evidence of the enduring relevance and usefulness of Bohr's phenomenological model in uncovering the mysteries of the atomic world.
In the complex tapestry of nuclear physics, the Bohr model serves as a guiding framework, illuminating the inner workings of atoms in the periodic table. Antimony, with its unique properties and atomic structure, provides a captivating subject for exploration within this model. By delving deeper into the electron configuration and valence properties of antimony, we gain insight not only into this fascinating element but also into the broader principles governing atomic behavior. In uncovering the mysteries of antimony through the Bohr model, we embark on a journey of discovery that transcends the boundaries of the microscopic world, enriching our understanding of the universe at its most fundamental level.
Comments
Post a Comment