The Lewis dot structure, a fundamental concept in chemistry, provides a visual representation of how atoms bond and arrange in molecules. In the case of iodine (I₂), understanding its Lewis dot structure provides insight into its chemical properties and behavior. As a halogen element, iodine exhibits unique characteristics that are reflected in its molecular structure. Let's delve deeper into the Lewis dot structure of iodine and uncover the intricacies of its bonding arrangement.
Understanding Lewis Dot Structure:
In the early 20th century, American chemist Gilbert N. The Lewis dot structure devised by Lewis involves drawing atoms as dots around a chemical symbol to depict valence electrons. Valence electrons are the electrons in the outermost energy level of an atom and are primarily responsible for combining with other atoms to form molecules. By representing valence electrons as dots, Lewis dot structures provide a simplified view of molecular bonding and geometry.
Electron configuration of iodine:
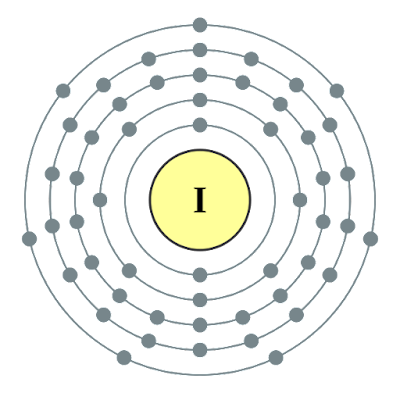
Before going deeper into the Lewis dot structure of iodine, it is necessary to understand its electron configuration. Iodine (I) belongs to group 17 (halogens) of the periodic table and its atomic number is 53. Its electron configuration is [Kr] 4d¹⁰ 5s² 5p⁵, indicating that iodine has seven valence electrons. These valence electrons play an important role in determining the chemical behavior and bonding pattern of iodine.
Lewis dot structure of iodine (I₂):
To construct the Lewis dot structure of iodine (I₂), we begin by denoting each iodine atom with its symbol (I) and placing seven dots around it to represent its seven valence electrons. Since iodine in its elemental state forms a diatomic molecule (I₂), we will explain how two iodine atoms join together to form a molecule.
In the Lewis dot structure of I₂, each iodine atom shares one electron with another iodine atom, forming a single covalent bond. As a result, the pair of iodine atoms share a total of two electrons between themselves, satisfying the octet rule for each iodine atom. The remaining five valence electrons on each iodine atom are represented as lone pairs.
The Lewis dot structure of iodine (I₂) can be represented as:
:I: :I:
\ /
∙∙∙
∙∙∙
∙∙∙
∙∙∙
/ \
:I: :I:
In this structure, two iodine atoms are linked by a single covalent bond, with each iodine atom surrounded by three lone pairs of electrons.
Chemical Implications:
The Lewis dot structure of iodine (I₂) shows its tendency to form covalent bonds with other iodine atoms, resulting in the formation of diatomic molecules. This covalent bonding behavior is characteristic of halogens, which usually exist as diatomic molecules in their elemental state.
Furthermore, understanding the Lewis dot structure of iodine provides insight into its reactivity and chemical properties. By sharing electrons in covalent bonds, iodine can participate in a variety of chemical reactions, including oxidation-reduction reactions and halogenation reactions.
Briefly, the Lewis dot structure of iodine (I₂) provides a visual representation of how iodine atoms join together to form diatomic molecules. Through the sharing of electrons in covalent bonds, iodine achieves a stable configuration, fulfilling the octet rule. By understanding the Lewis dot structure of iodine, chemists gain valuable insight into its binding behavior, reactivity, and role in chemical reactions.
Comments
Post a Comment